By watching the popular US television series “Forged in Fire”, viewers can learn the ins and outs of the bladesmith trade without getting up from the couch.
One of the secrets of a professional bladesmith is how to correctly harden the metal that they will hammer into a sharp and durable blade. This stage often becomes an insurmountable obstacle for the show’s participants. A bladesmith must master more than just the hammer and anvil. The most dramatic moment of the show is when a heated blank is dipped into a container of liquid. This is the hardening, which determines the quality of the surface, the characteristics of the steel, the shape of the blade, as well as the final assessment of the judges. After all, if a mistake is made, the metal can bend, crack or remain too soft.
Origins of hardening
Until the middle of the 19th century, it was believed that the quality of steel and steel products depended solely on hammering or blacksmithing. It was not until 1866-68, when the Russian scientist Dmitry Konstantinovich Chernov, who was studying the metal of defective guns, discovered and proved that high-quality steel is a product that has undergone heat treatment, including hardening, as a result of which changes occur in the metal, which could be mistaken for magic in the Middle Ages.
Steel has a crystalline structure, which tends to change depending on external conditions and form different stable crystal lattices. This feature is called crystal polymorphism and was first discovered by the German chemist Martin Heinrich Klaport in 1798 using calcium carbonate as an example.
Chernov advanced this theory in relation to steel. He specified four critical temperatures – a, b, c and d – which are known as Chernov’s points. When they are reached, the phase state and structure of the steel changes during cooling or heating of the solid steel product. Chernov’s discovery laid the foundation for the development of the science of metal heat treatment. After that, metallurgy generally began to apply an increasingly scientific approach and rely less on the experience accumulated by previous generations.
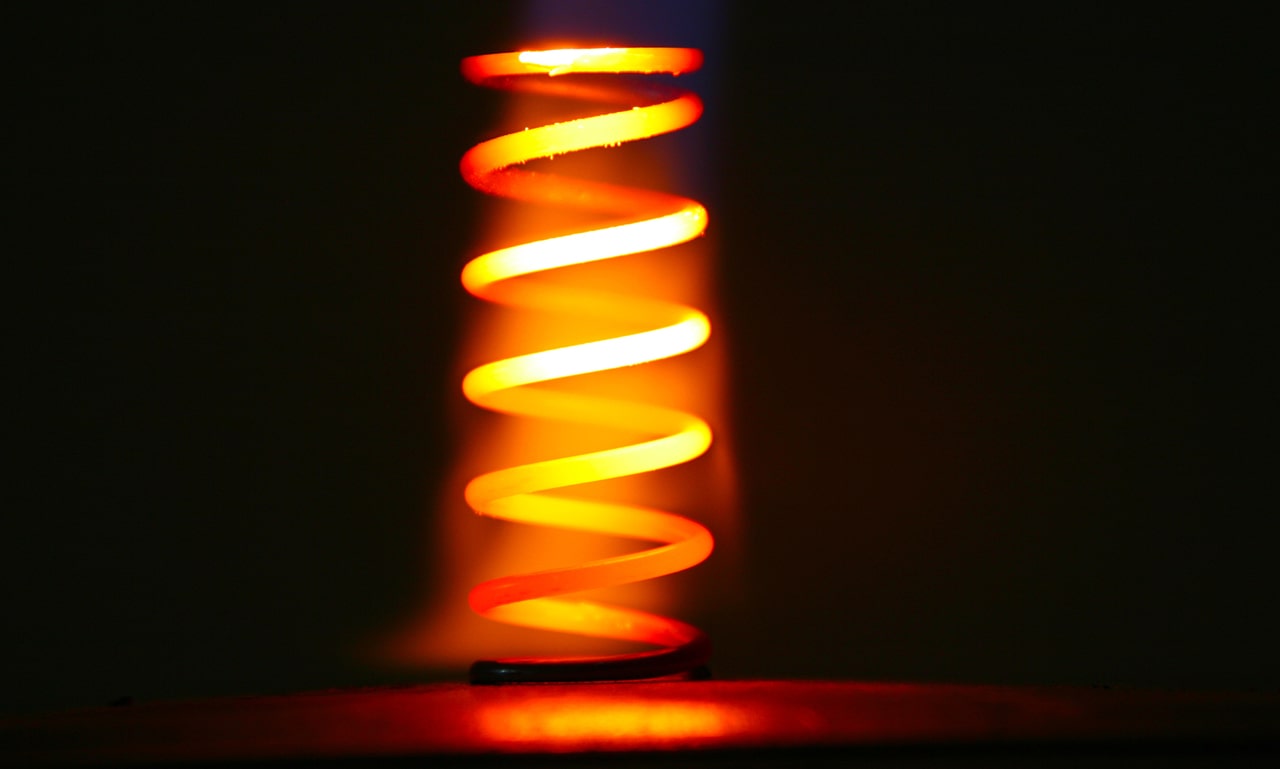
How hardening affects steel
At the molecular level, steel has a crystalline structure with polymorphic properties. The term ‘polymorphic’ is derived from the Ancient Greek words for ‘many’ and ‘shape’. In this case, the crystal lattices of steel – which can differ significantly from each other – transform one into another at a certain temperature. This is called a polymorphic transformation. In addition, under different cooling conditions (accelerated or, on the contrary, slow cooling), completely different phase components can form. The steel structure changes after hardening or other types of heat treatment. This process has an impact on the steel characteristics. Depending on the type and degree of the heat effect, the structures and phases obtained are called austenite, martensite, ferrite, cementite, pearlite and so on. Basically, these are physical and chemical terms with rather complex definitions.
Speaking in a language understandable to a wide audience, hardening is the high-temperature heating and rapid cooling of a steel product, which reduces the ductility and toughness of carbon steel. The material becomes strong, hard and brittle. This all happens in a solid state, that is, without heating the steel to the melting point.
Here is what it looks like in practice. A cold blank of a future knife or drill, for example, is heated to a point slightly above the critical temperature at which the very same polymorphic transformation of the crystal lattice occurs. The metal is held at that temperature for a certain amount of time. After that, the blank is quickly cooled in water, a salt solution or oil (depending on the degree of steel alloying and the required properties) to lock in the new crystal structure. Meanwhile, this creates internal stress in the steel product, which can lead to premature failure.
For this reason, in most cases the hardened part is subjected to another procedure: tempering. This is a technological process during which a steel product is heated to relatively low temperatures, followed by cooling in air or in a furnace. It helps to reduce the brittleness of the steel while maintaining its strength.
Steel hardening methods
One of the main factors that influence steel hardening is the medium in which it takes place. The choice of medium – including water or oil, as well as special polymer or salt solutions – determines the cooling rate. Each of these media has a certain cooling capacity, and if you choose an unsuitable one, the product either will not be hardened, or, on the contrary, excessive stresses will arise due to the excessive cooling rate, and the material will crack. Therefore, for each alloy, specific quenching liquids should be used: for example, water for carbon steels and oil for alloyed steels.
There are various methods for steel hardening, including in one medium, through the interrupted hardening method using two media, as well as stream hardening, step hardening and so on. The choice of method depends on many factors, including the original steel grade, the required final characteristics and the surface area to be hardened.
For example, in Japanese katana swords, only the cutting edge, called the hamon, is quenched. To achieve this, the bladesmiths coat an unhardened sword with a special type of clay, after which they wipe it off the edge of the blade and harden it. There have also been experiments. For example, using an infusion of hemp oil with green tea instead of water gave the katana blade a volumetric effect called a double hamon.
Steel hardening defects and imperfections
However, steel hardening is quite a delicate process. It is important to heat the product to the required temperature. As a rule, experienced blacksmiths determine the steel heating temperature visually, based on the colour of its surface. If the craftsman makes a mistake, the product will not attain the required characteristics. At industrial scale, the heating temperature is controlled by special devices, including pyrometers, thermocouples and other inspection equipment.
What can go wrong? The steel may not be hard enough. This is caused by a low heating temperature, short holding time or too slow cooling. Such a defect can be corrected by annealing and re-quenching. Overheating causes increased brittleness. It can also be corrected by annealing (normalising) and re-quenching.

Metal burning can occur when a steel product is heated to temperatures close to the melting point. This creates a very brittle metal and is a defect that cannot be corrected. Improper hardening also can lead to oxidation and decarburisation, deformation and cracking. One cause of such defects is an uneven structure of the parent metal or rate of temperature change. After all, the transition from one crystal structure to another (from austenite to martensite) leads to an increase in volume by 3%.
Therefore, to avoid or minimise such defects, special tables and colour charts are used. In addition, continuous-cooling transformation diagrams make it possible to select a specific steel grade to determine the correct heat treatment conditions to obtain the required structure.
There is a huge number of peculiarities that can arise during the heat treatment of steel products that must be accounted for in the technological process, which necessitates that serious study of metallurgy. Even television shows clearly demonstrate that in a resource-limited environment, both experience and knowledge offer great advantages to those who want to achieve the best results.
