Iron makes up 35% of Earth’s mass and 5.2% of the planet’s crust. This metal is one of the most important building blocks on Earth: the human body even contains 3 grams of iron.
The most stable element in the universe
Based on one hypothesis, iron appeared long ago in a distant galaxy as part of a nuclear fusion reaction inside a star. Immediately following the Big Bang, the heaviest elements were hydrogen, helium and lithium. In other words, there was no such thing as iron at first.
Then, according to the existing Big Bang model, the first clusters of matter started to form. They led to the creation of dense objects and, ultimately, the stars. It takes very high temperatures to transform lighter elements into iron through nuclear fusion and extreme pressure to generate such heat. The only places where such conditions exist are inside giant stars. It was in the stellar interior that silicon, sulphur, magnesium and other elements such as iron were formed. Elements heavier than iron were produced when giant stars exploded in a supernova. This is how stars came to be known as the universe’s iron factories.
Iron makes Earth suitable for life
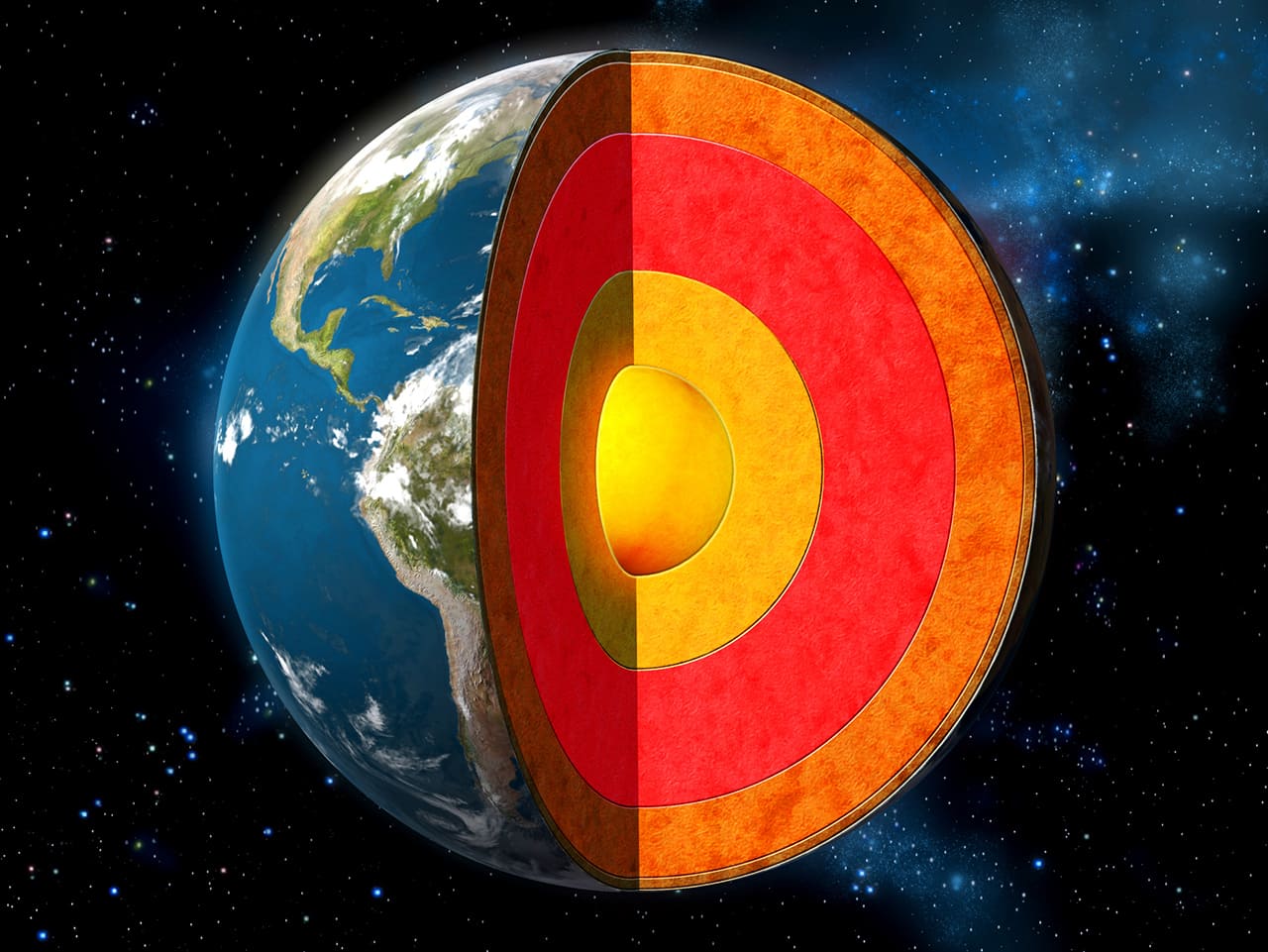
Iron accounts for a third of Earth’s mass. Most of it is in Earth’s core, rather than the crust. It exists as a liquid in the outer core and a solid in the inner core. In fact, Earth’s core is made up of 91% iron.
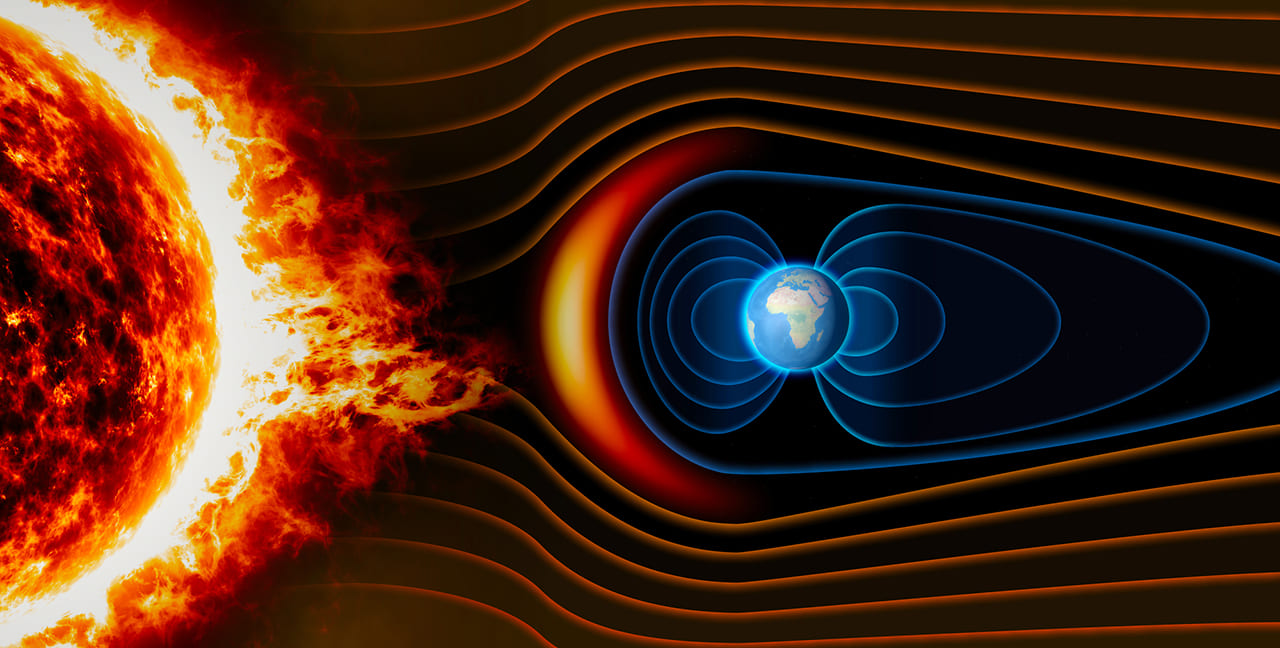
The iron in the outer core generates Earth’s magnetic field when it rotates together with the planet. While the strength of Earth’s magnetic field is insignificant compared with that of genuine magnets, it still plays a very important role. Compasses give us directions and help to distinguish north from south because of Earth’s magnetic field.
The iron in our planet’s core makes it suitable for life by forming a magnetic field that protects us from solar wind: plasma radiated by the upper atmospheric layer of the sun. Why is it so important? A flow of electrons and protons known as radiation is formed inside the plasma. The direct impact of cosmic radiation can: 1) change our DNA, which can cause cancer; 2) take away electrons from the atoms that make up our bodies; or 3) cause the atoms to absorb the radiation. All three scenarios would inevitably make life unstable. If there was no iron in Earth’s core, there would be no magnetic field to protect us, which would generally make Earth uninhabitable for us.
Three theories of iron’s discovery

Theory 1: mistake
The first theory is that iron was discovered by mistake. According to this theory, our ancestors mistook iron for chalcopyrite, a component of bronze that had a similar tone and colour. This theory is plausible only if we presume that our ancestors already possessed the technology for bronze production in the Bronze Age.
Theory 2: forest fire
According to this theory, a forest fire melted iron ore on Earth’s surface, which allowed our ancestors to discover iron, and use and shape oxidised iron ore.
The temperature of natural fire seldom exceeds 800°C, which is not enough to smelt iron ore. However, forest fires could have been much larger and lasted much longer in thick prehistoric jungles. This makes the forest fire theory plausible.

Theory 3: meteorites
Last, but not least, is the theory that humans discovered iron from fallen meteorites. Many of the meteorites that have fallen to Earth contain a large amount of iron, known as meteorite iron. Meteorites forming an alloy of iron and nickel are said to contain 4-20% nickel and 0.3-1.6% cobalt.
The most plausible of these theories is the first: that our ancestors mistook iron for bronze. Ancient documents and archaeological excavations indicate that humans first started to use iron around the fourth millennium BC in the region of Asia Minor. There is also evidence that iron purification technology existed around the third millennium BC in Mesopotamia and Egypt.
Regardless of its origins, we are indeed surrounded by iron today: from cars, vessels and planes to skyscrapers, household equipment and satellites. This most widespread of the universe’s stable elements will surely remain an important pillar that underpins much, if not all, of human civilisation for a long time to come.